Methane is the simplest hydrocarbon composed of a carbon atom bonded to four hydrogen atoms forming a tetrahedral structure. This stable molecule has a global warming potential around 86 times stronger per unit mass than CO2 on a 20-year time scale. Although methane’s emissions are less abundant than carbon dioxide emissions, it is important to address this problem.
Naturally, CH4 emissions occurs daily from methane’s earth cycle coming from wetlands, oceans, permafrost and underwater reserves. Recently, an active leak of methane of sea-bed was discovered in Antarctica rising concerns about this natural contribution of the already unbalanced atmosphere. However, anthropogenic activity has a much larger contribution as we will see next:
Agriculture and waste management sectors are the primary sources of methane emission counting for 227 Tg per year in 2017. This is equivalent to 277 million tons of methane released to the atmosphere (figure 1). Since farming involves the breeding of domestic livestock such as cattle, swine, sheep, and goats who produce CH4 as part of their normal digestive process. Also, when animal manure is stored or managed in lagoons or holding tanks, CH4 is produced. Methane is also generated in landfills as waste decomposes and in the treatment of wastewater. CH4 is also generated from domestic and industrial wastewater treatment and from composting.
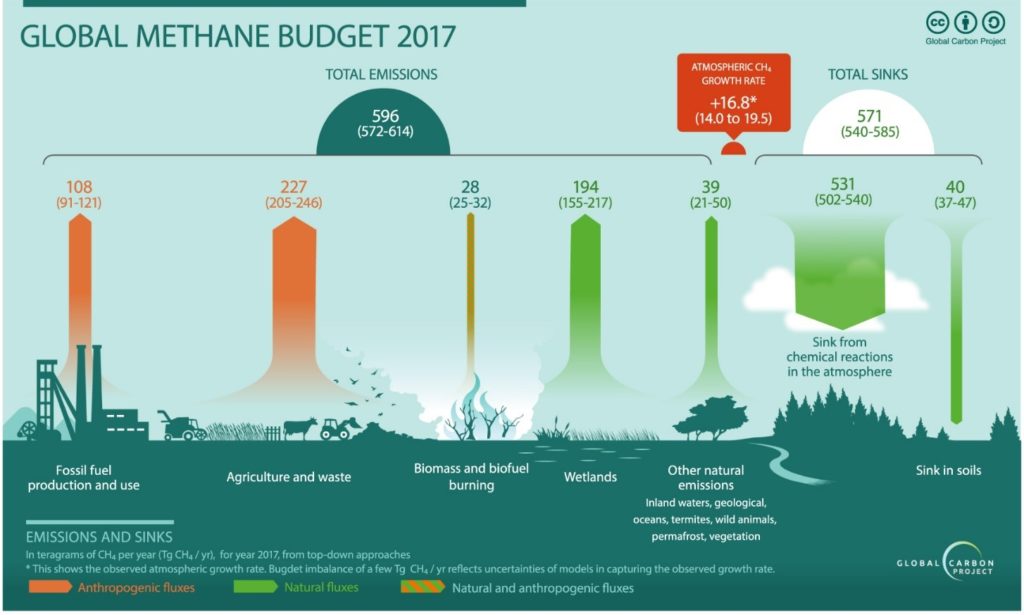
Figure 1. The global methane budget for year 2017 based on top-down methods for natural sources and sinks, anthropogenic sources, and mixed natural and anthropogenic sources. (extracted from ref. 1)
Fossil fuel and production also contributes to the overall emissions. Methane is the primary component of natural gas and is emitted to the atmosphere during the production, processing, storage, transmission, and distribution of natural gas and the production, refinement, transportation, and storage of crude oil. Coal mining is also a source of CH4 emissions.
Both sectors, agriculture and energy production, require active and strong mitigation actions. Agriculture requires a gentler and rationed production, we all want a nice grilled steak from time to time, right? Nevertheless, different approaches are taken to address methane conversion.
Generally, methane is converted to synthesis gas via steam reforming. The synthesis gas composed of H2/CO (best known as “syngas”) serves as a feedstock of many chemical processes, e.g., Fischer–Tropsch and methanol synthesis. However, the reaction conversion to syngas is highly endothermic and requires temperatures up to 900°C. By contrast, the partial oxidation of methane to synthesis gas can proceed to a more moderate temperature (around 500°C). Another approach is the utilization of CO2 for the oxidation of methane known as dry reforming. Carbon dioxide conversion releases atomic oxygen that serves for the oxidation of methane whilst methane serves as a source of hydrogens leading to the formation of syngas or C2 compounds. Also, this reaction is thermodynamically unfavorable but in this way two greenhouse gases are transformed to useful chemicals! Here is where catalysis has taken a step to promote the reaction at lower temperatures or increase the selectivity. Metal oxides as MgO or CeO2 in combination with metallic loadings of transition metals like Ni, Pt, Pd, etc. Nonetheless, this approach has significantly improved the outcome but recent studies have used catalytic process in conjunction with plasma or an electric field leading these reactions at a milder condition. Given previous results using low temperature plasmas like Dielectric Barrier Discharges, cold plasmas are a promising route for methane conversion to a sustainable chemical industry.
This text was written by Carolina Garcia
- Jackson, R. B., Saunois, M., Bousquet, P., Canadell, J. G., Poulter, B., Stavert, A. R., … & Tsuruta, A. (2020). Increasing anthropogenic methane emissions arise equally from agricultural and fossil fuel sources. Environmental Research Letters, 15(7), 071002.
- Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990-2015. (2020, September 11). US EPA. https://www.epa.gov/ghgemissions/inventory-us-greenhouse-gas-emissions-and-sinks-1990-2015

